Panel 1
Emiliano Panieri, Debora Romoli
Over the last hundred years, global chemical production has grown exponentially, from 1 million tonnes in 1930 to several hundred million tonnes today. The European Union (EU) is the second largest producer in the world after China, and it is estimated that over 100,000 chemical substances are present on the European market. Italy, with a production value exceeding 66 billion euros, is the third largest producer in Europe after Germany and France, and the twelfth globally. Around 2,800 chemical companies operate in Italy, employing more than 112,000 people, although chemical products are used in all production sectors.
The European regulation on chemical substances is likely the most ambitious in the world, aiming to ensure a high level of protection for human health and the environment. The main reference regulations are REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) and CLP (Classification, Labelling and Packaging). Responsibility for the safe management of chemicals lies primarily with the companies that manufacture, import, or use them. The European Chemicals Agency (ECHA) and the competent authorities of EU Member States monitor company compliance and can intervene with specific measures if risks to human health or the environment are not properly controlled. Through regulatory process monitoring, the indicator provides elements to assess progress in achieving safety objectives set by the legislation.
Chemical substances in Europe are regulated through comprehensive strategies and sector-specific legislation. The REACH regulation applies to all sectors that deal with chemical substances throughout their entire life cycle. Its goal is to ensure a high level of protection for human health and the environment, while also promoting the competitiveness and innovation of the European chemical industry, with a strong commitment to the use of alternatives to animal testing.
The CLP regulation ensures that the hazards of chemical substances are clearly communicated through classification and labelling across the entire supply chain. The indicator measures the effectiveness of the main EU regulatory processes responsible for chemical safety: registration, dossier evaluation, prioritised substance evaluation, and risk management measures at the European level (harmonised classification, restrictions, authorisation).
The purpose is to monitor the regulation’s ability to achieve its core objective: raising safety levels in the management of chemicals throughout all stages of their life cycle.
Twenty years after the first strategic approach to chemical management in Europe (White Paper: Strategy for a Future Chemicals Policy), the European Union has outlined a new long-term chemical strategy. Aligned with the Green Deal, the EU’s strategy aims to create a toxic-free environment in which chemicals are produced and used in ways that maximise their societal contribution while avoiding harm to the environment and current and future generations.
The EU has one of the most comprehensive and protective regulatory frameworks for chemicals, encompassing around forty legislative instruments. Among them, REACH and CLP are the two core horizontal regulations applying to all chemical substances, complemented by sector-specific regulations for certain product types (e.g., plant protection products, biocides, cosmetics).
Since 2007, REACH Regulation (EC No 1907/2006), aims to close knowledge gaps about chemicals, promote the substitution of substances of high concern with safer alternatives, and improve risk management. It does this primarily by placing the burden of safety on companies, which are responsible for manufacturing, placing on the market, or using chemicals in a way that does not harm human health or the environment.
CLP Regulation (EC No 1272/2008), since January 2009, governs the classification, labelling, and packaging of hazardous substances and mixtures. Based on the United Nations’ Globally Harmonised System (GHS), its aim is to ensure a high level of health and environmental protection and enable the free movement of chemicals across the EU market.
REACH establishes a unified risk management system, which includes:
- Registration of all substances produced or imported in quantities greater than 1 tonne per year;
- Evaluation of registration dossiers;
- Evaluation of priority substances based on volume and hazardous properties;
- Risk management measures at EU level, such as restrictions and authorisations, for substances presenting unacceptable risks.
Since 2016, based on early implementation experience, ECHA has developed an integrated regulatory strategy to unify various regulatory processes. The strategy aims to:
- Efficiently identify substances or substance groups of concern and generate data needed for safety assessment, enabling timely risk management actions;
- Ensure appropriate and timely interventions by all actors (ECHA, Member States, European Commission, and industry) across REACH and CLP processes;
- Provide stakeholders and the public with assurance that companies meet REACH information requirements and improve communication on safe use along the supply chain.
Panel 2
EU, Regulation (EC) No 1907/2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH)
EU, Regulation (EC) No 1272/2008 on the classification, labelling and packaging of substances and mixtures (CLP)
CEFIC, 2023, Facts and Figures of the European Chemical Industry
ECHA, https://echa.europa.eu/home
ECHA, 2024a, Annual Report 2023, ISBN 978-92-9468-355-7
ECHA, 2024b, Progress in Substance Evaluation 2012–2023 (https://echa.europa.eu/progress-in-substance-evaluation)
Federchimica, 2023, The Chemical Industry in Figures 2023
EU, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions – Chemicals Strategy for Sustainability
EU, Towards a Toxic-Free Environment, COM(2020) 667 final
Absence of reference values
None
Data quality assessment
Cefic - The European Chemical Industry Council
ECHA - European Chemicals Agency
Federchimica
CEFIC 2023_Facts_and_Figures_The_Leaflet. Data files xls (https://cefic.org/a-pillar-of-the-european-economy/facts-and-figures-of-the-european-chemical-industry/)
Federchimica l'industria chimica in cifre-2023 (https://www.federchimica.it/docs/default-source/materiali-assemblea-2023/pubblicazioni/l'industria-chimica-in-cifre-2023.pdf?sfvrsn=11e5093_4)
REACH registration statistics, Data as of: 31/12/2023 (https://echa.europa.eu/it/registration-statistics)
ECHA Substance Evaluation table (https://echa.europa.eu/it/information-on-chemicals/evaluation/community-rolling-action-plan/corap-table)
ECHA, Banca dati dell'Inventario delle Classificazioni e delle Etichettature (https://echa.europa.eu/it/information-on-chemicals/cl-inventory-database)
ECHA, Elenco delle sostanze estremamente preoccupanti candidate all’autorizzazione (https://echa.europa.eu/it/candidate-list-table)
ECHA, Elenco di restrizioni (https://echa.europa.eu/it/substances-restricted-under-reach)
ECHA, Elenco di autorizzazioni (https://echa.europa.eu/it/authorisation-list)
ECHA, Andamento della valutazione (https://echa.europa.eu/it/overall-progress-in-evaluation)
ECHA, Registry of restriction intentions until outcome (https://echa.europa.eu/it/registry-of-restriction-intentions)
ECHA, Annual Report 2023, ISBN: 978-92-9468-355-7 (https://echa.europa.eu/documents/10162/7362407/annual_Report_2023_en.pdf/ca16f5a4-a8ae-ac22-21f1-57af476f0bb1?t=1713865234883)
National
2008-2023
Indicator assessment
Data are collected from reference sources and represented either as point data (for specific years) or cumulative data (for specific time periods). In some cases, processing required calculations and/or Boolean operators.
Key regulatory processes for chemical safety:
- Registration of substances produced or imported and placed on the European market above 1 tonne/year;
- Evaluation of registration dossiers;
- Evaluation of priority substances;
- EU-level risk management measures (harmonised classification, restrictions, authorisations).
Through registration, companies submit dossiers to ECHA containing substance properties, uses, and implemented risk controls. Classifications made by companies (self-classifications) are shared with ECHA and published in the Classification and Labelling Inventory. This inventory serves as the main reference for harmonised classification and promotes convergence of classifications among companies. The information is also used by Member States to identify potentially hazardous substances.
ECHA checks dossiers for completeness and adequacy and examines testing proposals to avoid unnecessary animal testing.
The combined action of REACH and CLP ensures that each year a number of substances are regulated to improve human and environmental safety. However, due to the very high number of chemicals on the EU market, only a portion of them can be addressed annually.
From 2012 to 2023, 321 substances (ECHA, 2024b) were evaluated to identify hazardous properties under relevant exposure conditions. About 70% of evaluations resulted in requests for more data. From 2009 to 2023, ECHA issued 4,170 decisions relating to 13,780 data requests (Table 3). Since REACH came into force, 235 substances have been added to the Candidate List for authorisation (Figure 6), and 53 restriction proposals have been submitted (Table 1). Additionally, around 570 opinions on harmonised classification proposals have been adopted by ECHA’s Risk Assessment Committee (Table 2).
The number of regulated chemical substances has steadily increased, indicating a positive trend in chemical safety. However, it remains difficult to estimate when the ambitious goal of regulating all problematic substances on the EU market will be achieved.
Overall, chemical safety in terms of human and environmental impact is improving, reflecting the growing commitment to implementing REACH and CLP risk management measures. This is evident in the increasing number of registered substances and regulatory processes from 2009 to 2023.
Data
Table 1: Restriction proposals and decisions (2010–2023)
ISPRA elaboration based on ECHA data
Table 2: Number of CLH opinions per year (2009–2023)
ISPRA elaboration based on ECHA data
Table 3: Progress of Evaluation Processes (2009–2023)
ECHA
(https://echa.europa.eu/it/overall-progress-in-evaluation consultata on 5/11/2024)
(*) The substance evaluation process began in 2012





Thousands of chemical substances are produced, consumed, and traded worldwide. The chemical sector plays a significant role in the global economy, showing consistent growth trends, albeit affected by the 2008 global economic crisis and the 2020 health crisis. In 2022, global chemical production was valued at €5,622 billion. With a 43% share amounting to €2,390 billion, China is the world’s leading producer. The European chemical industry ranks second with €764 billion and a 14% share (Figure 1).
Italy, with a production value of over €66 billion in 2022, remains the third-largest producer in Europe—after Germany and France—and twelfth globally. Around 2,800 chemical companies operate in Italy, employing over 112,000 people (Federchimica, 2023). Italian chemical production is primarily concentrated in three sectors:
Basic chemicals and fibres, accounting for about 40% of Italy’s chemical production value, produce key components for downstream industries. This includes petrochemical products and derivatives, basic inorganics (chlorine, soda, sulphuric acid), chemical fibres, fertilizers, dyes and pigments, and technical gases.
Fine and specialty chemicals, representing almost 44% of the total, include auxiliary products for industry, paints and inks, and plant protection products, serving as intermediates for other industrial sectors.
Consumer chemicals, making up over 16%, include chemicals intended for final consumers such as specific paints and coatings, detergents, cosmetics, and fragrances.
Although chemical activity is distributed across the entire country, there is a higher concentration in Northern Italy, which accounts for about 77% of sector employment (almost 41% in Lombardy), compared to 12% in Central Italy and around 10% in the South (Federchimica, 2023).
The registration of chemical substances produced or imported above one tonne per year is the first and most important requirement under the REACH Regulation to ensure the safe use of chemicals. From the entry into force of REACH up to 31 December 2023, 104,945 registration dossiers concerning 22,566 substances were submitted to the European Chemicals Agency (ECHA) (Figure 2). Most registrations were submitted by companies based in Germany (28%), France (11%), and the Netherlands (10%). Italy, with 9,590 registrations (9%) covering 4,955 substances, ranks fourth among EU Member States (Figures 3 and 4).
ECHA uses the information provided in registration dossiers to build a database that also supports other regulatory processes. The goal is both to define appropriate risk management measures and to make chemical substance information publicly accessible. These data are fundamental for companies in preparing safety data sheets and communicating safe use conditions along the supply chain. Therefore, ensuring the quality of registration dossiers is crucial, which means information must be compliant, complete, and easily accessible.
ECHA conducts two types of dossier evaluation to ensure compliance:
Compliance Check (CCH) (Article 41)
Examination of Testing Proposals (TPE) (Article 40)
From 2009 to 2023, ECHA, with support from Member States, conducted 4,072 compliance checks and 2,319 testing proposal evaluations. In cases of non-compliance or for all TPEs, ECHA sends a request for additional information to the registrant. Compliance checks assess:
the completeness and adequacy of submitted information;
the accuracy of the chemical safety assessment;
the appropriateness of risk management measures.
Compliance checks are primarily targeted at substances (or groups of substances) that raise potential concern due to their hazard or exposure, where improving data quality can significantly enhance safety.
Testing proposals are mandatory for registrations above 100 tonnes/year for data required under Annexes IX and X of REACH. ECHA reviews all proposals with the aim of avoiding unnecessary testing, especially on vertebrate animals, which is only allowed when absolutely necessary and as a last resort. Since 2009, ECHA has issued 2,381 compliance decisions covering 9,959 information requirements due to identified data gaps. Additionally, it adopted 1,589 decisions on testing proposals, addressing 3,109 information requirements (Table 3).
Substances registered in large volumes or with hazardous properties undergo further evaluation by Member State competent authorities as part of the Community Rolling Action Plan (CoRAP), coordinated by ECHA over a three-year period. Priority criteria include hazard, exposure, and total volume on the market. The evaluation aims to confirm (or dismiss) initial grounds for concern and identify appropriate risk management measures. It involves a thorough review of the registrants' chemical safety reports, may request further information, and concludes with an evaluation report. Under the previous legislation, only about 140 substances were evaluated over twenty years.
Under REACH, 321 substances were evaluated between 2012 and 2023 for properties such as carcinogenicity, mutagenicity, reproductive toxicity, sensitisation, PBT (persistence, bioaccumulation and toxicity), and endocrine disruption, especially under relevant exposure conditions (ECHA, 2023b). About 70% of these evaluations led to additional information requests, confirming the validity of the initial concerns for substances included in CoRAP up to the 2024–2026 period (Figure 5).
The evaluation process is also key to implementing REACH's authorisation goals. It was crucial in meeting the objectives of the SVHC Roadmap, which aimed to include all known Substances of Very High Concern (SVHCs) on the Candidate List by the end of 2020. This work continues under the Integrated Regulatory Strategy, which seeks to assign risk management priorities and/or data generation needs for all registered substances by 2027.
Substances on the Candidate List may be added to Annex XIV (Authorisation List). Once listed, these substances may no longer be placed on the market or used after a certain date unless specific authorisation is granted. Through the authorisation process, REACH ensures that SVHCs are gradually replaced by less hazardous substances or technologies.
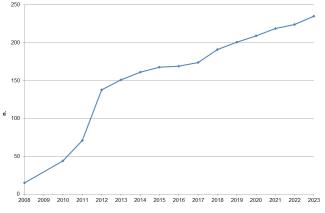
Since 2008, 235 substances or substance groups have been identified as SVHCs and added to the Candidate List (Figure 6), and 59 substances have been included in Annex XIV. Annex XVII contains all adopted restrictions, including those from the previous Directive 76/769/EEC. Thus, restrictions have served as a risk management tool for over forty years.
As of 31 December 2023, Annex XVII included 77 entries referring to substances or substance groups subject to restriction, some of which include numerous chemicals. Since the entry into force of REACH, the number of restriction proposals submitted by Member States and ECHA has reached 530 (Table 1).
From 2009 to 2023, ECHA’s Risk Assessment Committee adopted about 570 opinions on harmonised classification and labelling (CLH) proposals (Table 2), while the Classification and Labelling Inventory contains data on the classification and labelling of approximately 270,000 substances.