Panel 1
Valeria Giovannelli, Matteo Lener, Giovanni Staiano
The pressure indicator reports the number and scale, and characteristics of authorized (field trials) of Genetically Modified Plants (GMPs) in Italy—pursuant to Directive 2001/18/EC—since 1999. The indicator is populated using data produced by the Ministry of the Environment and Energy Security.
Field trials experienced a sharp decline by 2000, and no new trials were authorized from 2001 onward. The last ongoing trials, initially granted a multi-year authorizations, concluded in 2010.
Following the introduction of Law No 68 of 13th June 2023, 2 field trials involving genome-edited rice and grapevine—designed for pathogen resistance—were authorized in 2024.
Pressure indicator referred to the number and extent of experimental releases of Genetically Modified Plants (GMPs) present on Italy from 1999 to december 2024.
To quantify the number of deliberate experimental releases of Genetically Modified Plants (GMPs) into natural and agricultural ecosystems.
Directive (EU) 2001/18 (repealing Council Directive 90/200/EEC);
Regulation (EC) No 1829/2003;
Legislative Decree No. 224/2003;
Ministerial Decree of 19 January 2005;
Directive (EU) 2015/412;
Legislative Decree No. 227/2016;
Ministerial Decree of 8 November 2017;
Ministerial Decree No. 58 of 1 March 2018;
Law No. 68 of 13 June 2023.
Field trials of GMPs for in Italy are authorised under Legislative Decree No. 224 of 8 July 2003, which implements Directive 2001/18/EC on the deliberate release into the environment of genetically modified organisms (GMOs). In accordance with the Directive, the decree establishes that, prior to authorizing any GMO’ release, an environmental and human health risk assessment must be carried out to verify its safety.
Legislative Decree No. 224/2003 designates the Ministry of the Environment and Protection of Land and Sea as the competent national authority and establishes the Register of GMOs release sites, both for experimental and commercial purposes.
The Law No. 68 of 13 June 2023 stipulates that, until 31 December 2025, experimental releases of GMOs produced using genome editing techniques (site-directed mutagenesis or cisgenesis) are exempted from the risk assessment for agrobiodiversity, farming systems and the agri-food chain, as well as from the requirements laid down by Ministerial Decree of 19 January 2005.
In accordance with Article 32 of the Legislative Decree No. 224/2003, the Ministerial Decree of 8 November 2017 adopted the general surveillance plan for the deliberate release into the environment of GMOs. The plan defines the objective of scope of the different inspection activities foreseen, the coordination mechanisms between the different competent authorities, and the criteria and procedures for updating every 4 years, if needed. The general surveillance plan is implemented by an Operative National plan which identifies and describes the number and scope of inspections to be conducted annually. This decree also establishes the National Register of Inspectors, who act as judicial police officers during inspections.
In 2018, ISPRA (Italian Institute for Environmental Protection and Research), pursuant to Ministerial Decree No. 58 of 1 March 2018, was assigned the advisory and technical support functions to the competent authority, previously held by the Interministerial Evaluation Committee (pursuant to Article 2 of Legislative Decree No. 224/2003).
Panel 2
Data quality assessment
MASE (Ministero dell'ambiente e della Sicurezza Energetica)
MASE, Registro dei rilasci ambientali OGM (https://bch.mase.gov.it/index.php/it/?view=article&id=435&catid=15)
Italy
1999 - 2024
Indicator assessment
The indicator, which does not involve complex calculations, was designed as a review of the main data concerning field trials of Genetically Modified Plants (GMPs) in Italy, from 1999 to 2024.
In 2024, two new deliberate experimental releases of genetically modified plants were authorised, putting an end to a suspension that had been in place since 2010.
Since 2005, no authorizations have been requested in Italy for new field trials due to the lack of publication of operational technical protocols for risk management of individual GM species, as required by Article 1, paragraph 2 of the Ministerial Decree of 19 January 2005, "Prescriptions for the risk assessment of agrobiodiversity, farming systems and the agri-food chain related to deliberate releases into the environment of GMOs for purposes other than placing on the market."
A marked reduction in the number of field trials was already observed starting in 2000, and since 2005 only field trials with multi-year authorizations granted under the legislation preceding Legislative Decree 224/2003 have been conducted. These trials ended in 2009, and consequently, from 2010 there were no ongoing field trials in Italy.
In 2024, following the introduction of Law No. 68 of 3 June 2023, two new experimental trials were authorized.
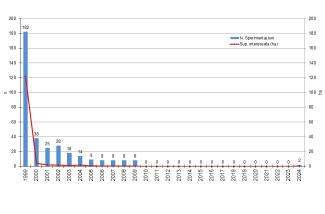
In 1999, the highest number of trials was recorded, totaling 182, covering approximately 122 hectares. Starting from 2000, a marked decline in the number of trials was observed, and from 2005 to 2009 only multi-year authorized trials remained in the field. Due to regulatory reasons, trials ceased from 2010 onwards, resuming again in 2024 following the introduction of Law No. 68 of 3 June 2023 (Figure 1).